CH105 Chapter 5 Introduction to Organic Chemistry Chemistry
Chirality and Stereoisomers Chemistry LibreTexts
There are two enantiomers of glucose, called D-glucose and L-glucose. The D-enantiomer is the common sugar that our bodies use for energy. It has n = 4 stereocenters, so therefore there are 2 n = 2 4 = 16 possible stereoisomers (including D-glucose itself). In L-glucose, all of the stereocenters are inverted relative to D-glucose. That leaves.
PPT Isomerism Recap PowerPoint Presentation, free download ID397852
Although sugar enantiomers may display the same or similar biological activity, it is obvious that the protein-binding properties of a d -sugar component of a lead synthetic glycoside are different from its L-enantiomer (or vice versa).

Enantiomers in nature CheMystery
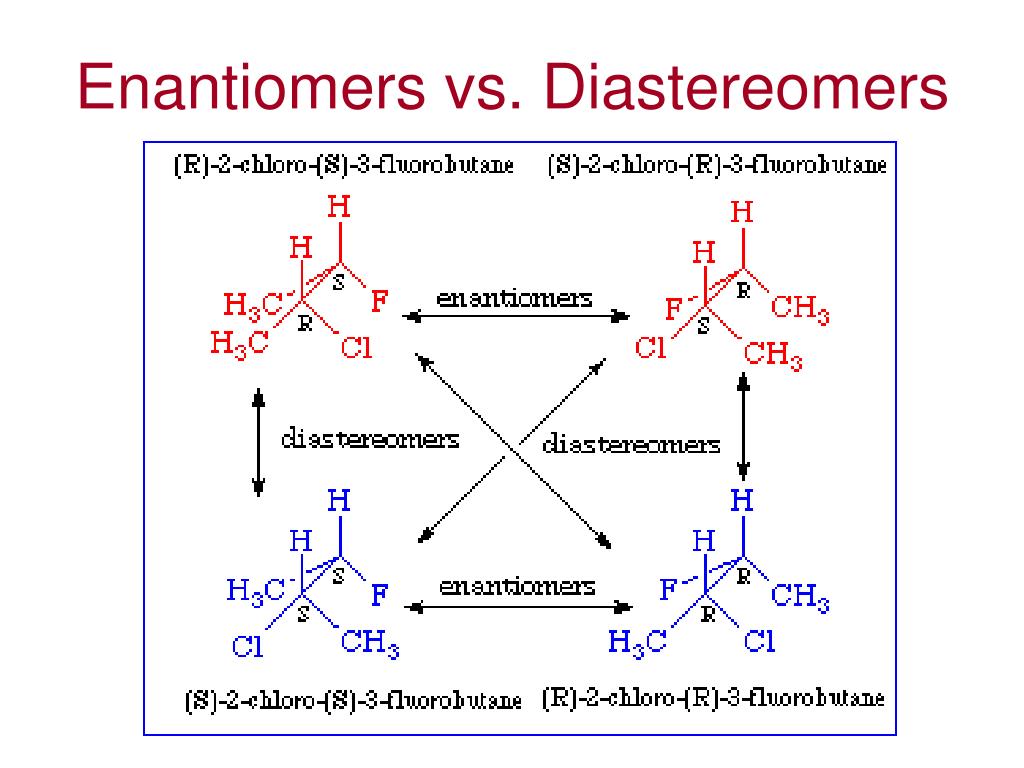
1 Answer Maxwell Aug 12, 2016 They are not enantiomers. They are diastereomers. Explanation: Diastereomers are molecules that have 2 or more stereogenic centers and differ at some of these centers with respect to absolute configurations. This disqualifies them from being mirror images of each other.

Adisi Nukleofilik
Enantiomers are a pair of molecules that exist in two forms that can not be superimposed on each other but are mirror images of each other. They have a chiral carbon which is a center of carbon.
:max_bytes(150000):strip_icc()/chirality-diagram-56a12ca45f9b58b7d0bcc7b5.png)
What is an Enantiomer?
Their enantiomers were given the same name with the introduction of systematic nomenclatures, taking into account absolute stereochemistry (e.g. Fischer nomenclature, d / l nomenclature). For the discovery of the metabolism of glucose Otto Meyerhof received the Nobel Prize in Physiology or Medicine in 1922. [16]

Epimers and Anomers Chemistry Steps
There are two enantiomers of glucose, called D-glucose and L-glucose. The D-enantiomer is the common sugar that our bodies use for energy. It has n = 4 stereocenters, so therefore there are 2 n = 2 4 = 16 possible stereoisomers (including D-glucose itself). In L-glucose, all of the stereocenters are inverted relative to D-glucose. That leaves.
5.8 Diastereomers Chemistry LibreTexts
Enzyme-free substrate is used for SERS sensing glucose enantiomers. • Au NPs play as the oxidase mimics instead of the SERS substrate. • Intrinsic structure of MOFs is favorable for applying as nanoreactors. • Enantioselective identification is achieved via onsite growth of Prussian blue. • This platform is also useful for other.
Chiral responsive CdotsAu NP complex towards glucose enantiomers. (a)... Download Scientific
For example, let's consider the glucose molecule in its open-chain form (recall that many sugar molecules can exist in either an open-chain or a cyclic form). There are two enantiomers of glucose, called D-glucose and L-glucose. The D-enantiomer is the common sugar that our bodies use for energy. It has n = 4 stereocenters, so therefore.

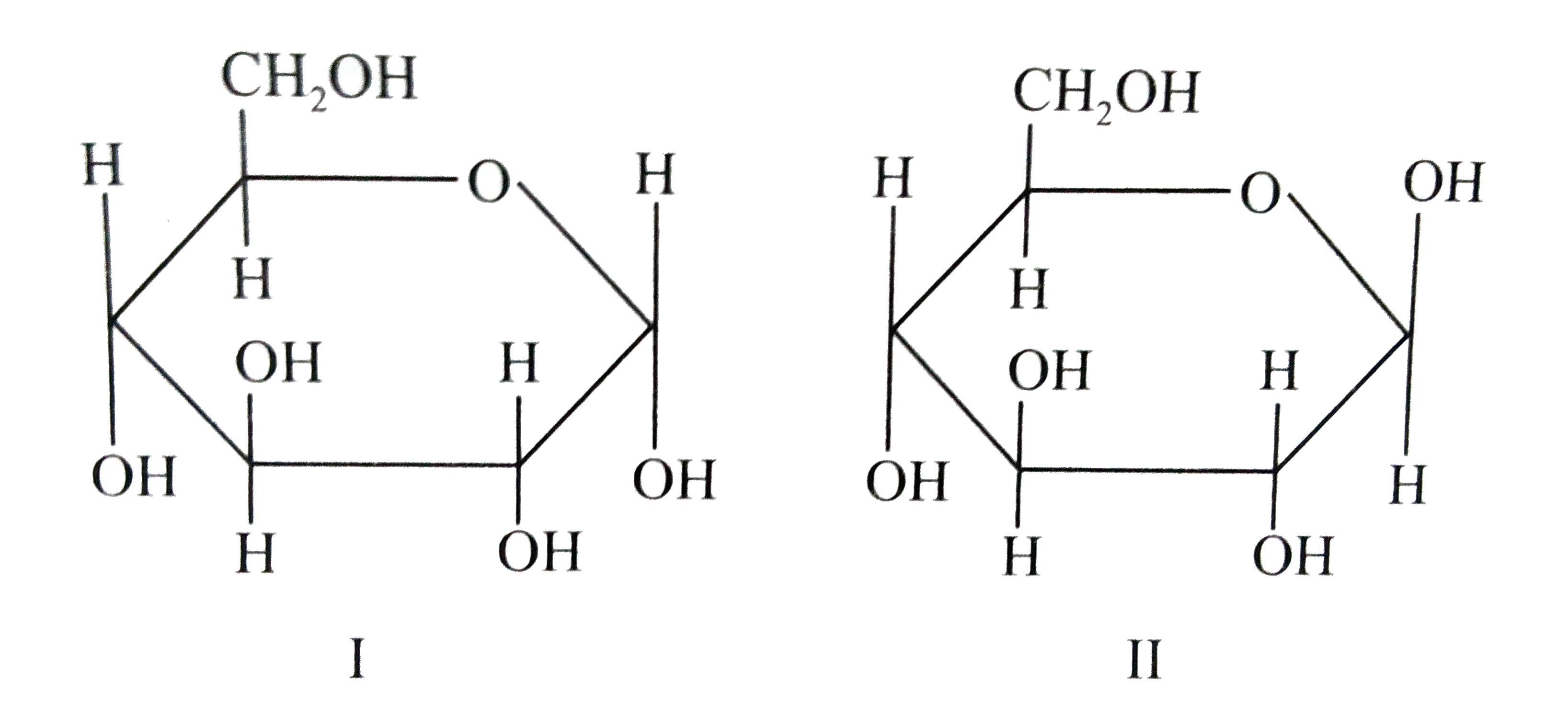
ɑDglucoses and βDglucose are anomersdiastereomers that differ in only one chiral center D
There are two enantiomers of glucose, called D-glucose and L-glucose. The D-enantiomer is the common sugar that our bodies use for energy. It has n = 4 stereocenters, so therefore there are 2 n = 2 4 = 16 possible stereoisomers (including D-glucose itself). In L-glucose, all of the stereocenters are inverted relative to D-glucose. That leaves.
Alpha D Glucose and Beta D Glucose Are Enantiomers
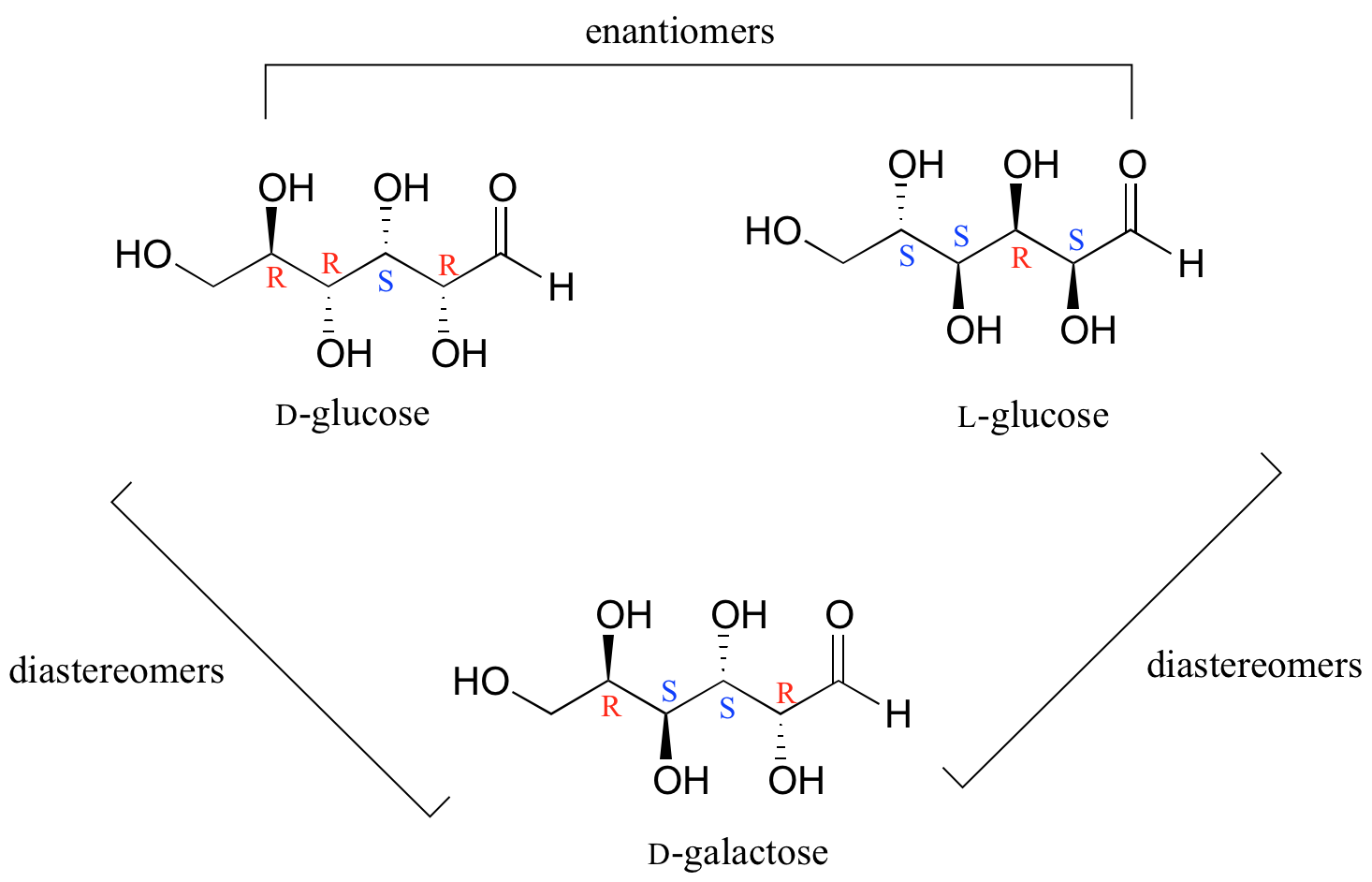
Q1 What are Epimers with examples? Epimers are carbohydrates that differ in the location of the -OH group in one location. Both D-glucose and D-galactose are the best examples. D-glucose and D-galactose epimers create a single difference at C-4 carbon. They are not enantiomers, they are just epimers, or diastereomers, or isomers. Q2

D and LGlucose are enantiomers. Chemistry, Chemistry notes, Science chemistry
There are three common naming conventions for specifying one of the two enantiomers (the absolute configuration) of a given chiral molecule: the R/S system is based on the geometry of the molecule; the (+)- and (−)- system (also written using the obsolete equivalents d- and l-) is based on its optical rotation properties; and the D/L system is based on the molecule's relationship to.
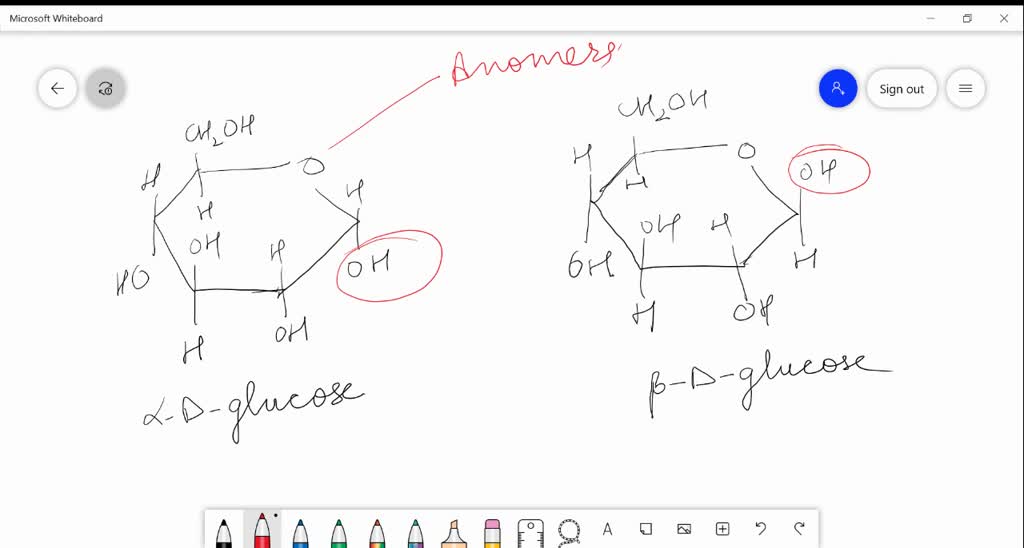
SOLVEDαDglucose and βDglucose are (a) anomers (b) C2 epimers (c) C3 epimers (d) enantiomers
D- and L-is an old but still-convenient shorthand for saying that molecules are enantiomers. e.g. D-glucose and L-glucose are non-superimposable mirror images without having to write out a long IUPAC name with lots of ( R) and ( S) descriptors. Most natural sugars are D- and most natural amino acids are L- .
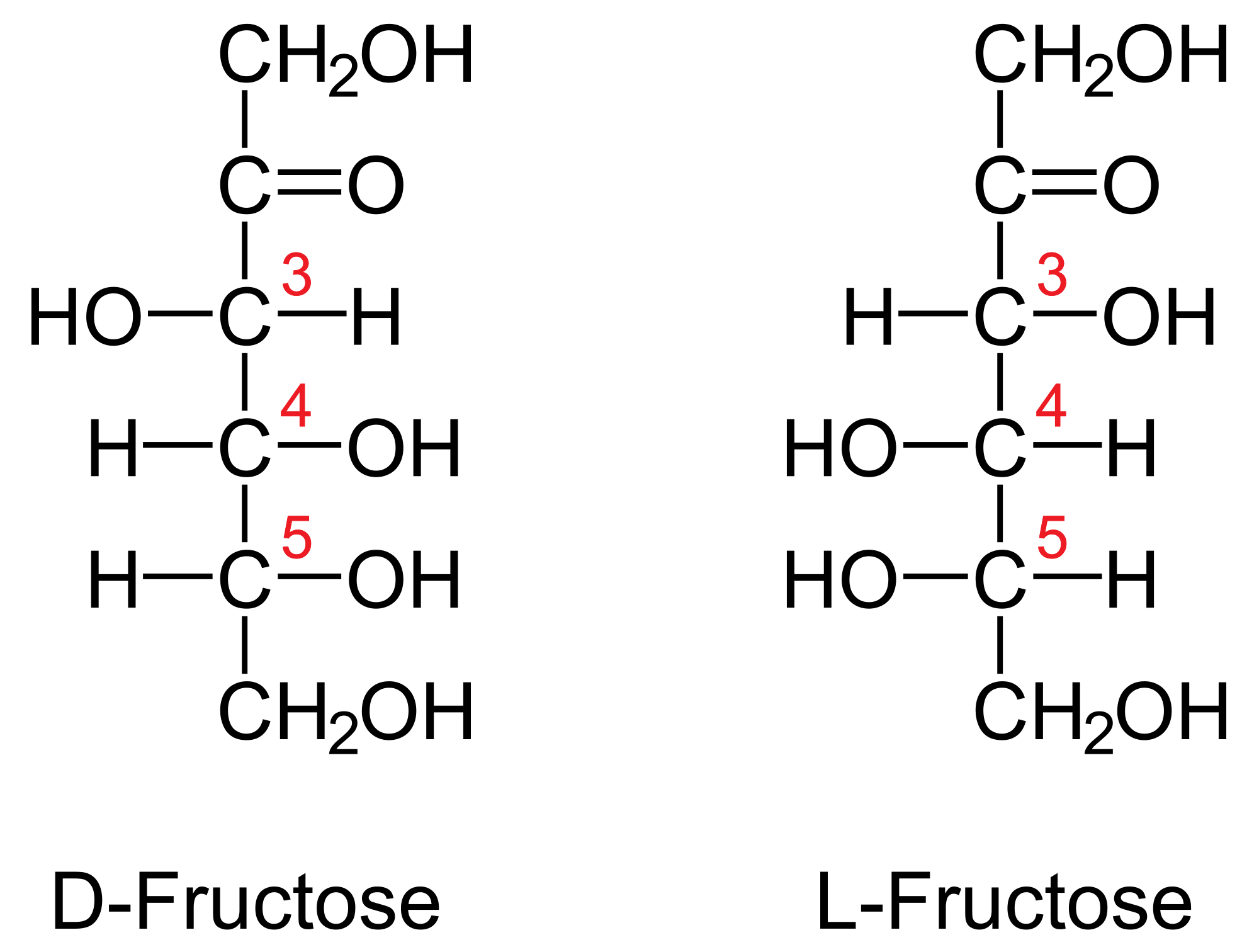
Glucose And Fructose
The D- and L-glucose are true enantiomers. So, enantiomers, which means that they're complete mirror images. They differ at every single chiral carbon. Now that being said, if the D-aldohexoses, these glucose, if the D- and L-aldohexoses are enantiomers, that means that all of the D-aldohexoses have to be diastereomers of each other, because.
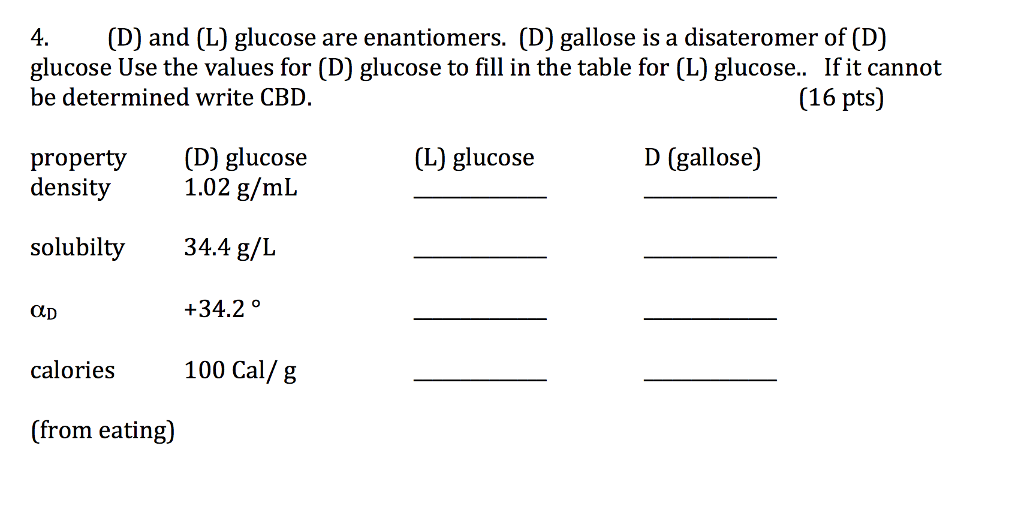
Solved (D) and (L) glucose are enantiomers. (D) gallose is a
l -Glucose does not occur naturally in living organisms, but can be synthesized in the laboratory. l -Glucose is indistinguishable in taste from d -glucose, [1] but cannot be used by living organisms as a source of energy because it cannot be phosphorylated by hexokinase, the first enzyme in the glycolysis pathway.

Enantiomers Of Alpha D Glucose Johnathon Howells
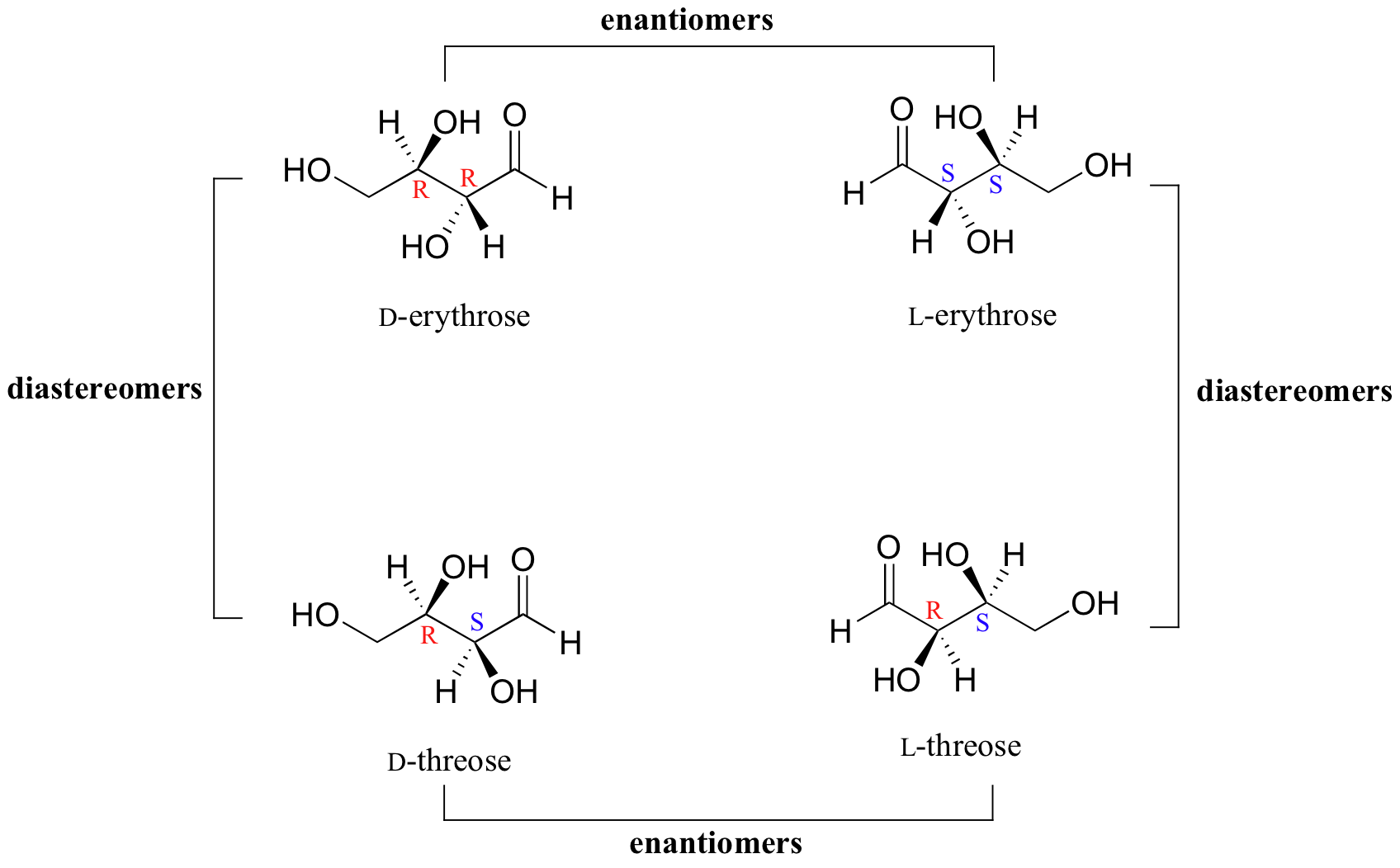
Thus, L-glucose and D-glucose are enantiomers, but D-Erythrose and D-Threose are diastereomers. Figure \(\PageIndex{1}\): Diastereomers. Figure \(\PageIndex{2}\): Enantiomers. Sugars of 5-7 carbons can fairly easily form ring structures (called Haworth structures). For aldoses like glucose, this involves formation of a hemi-acetal.
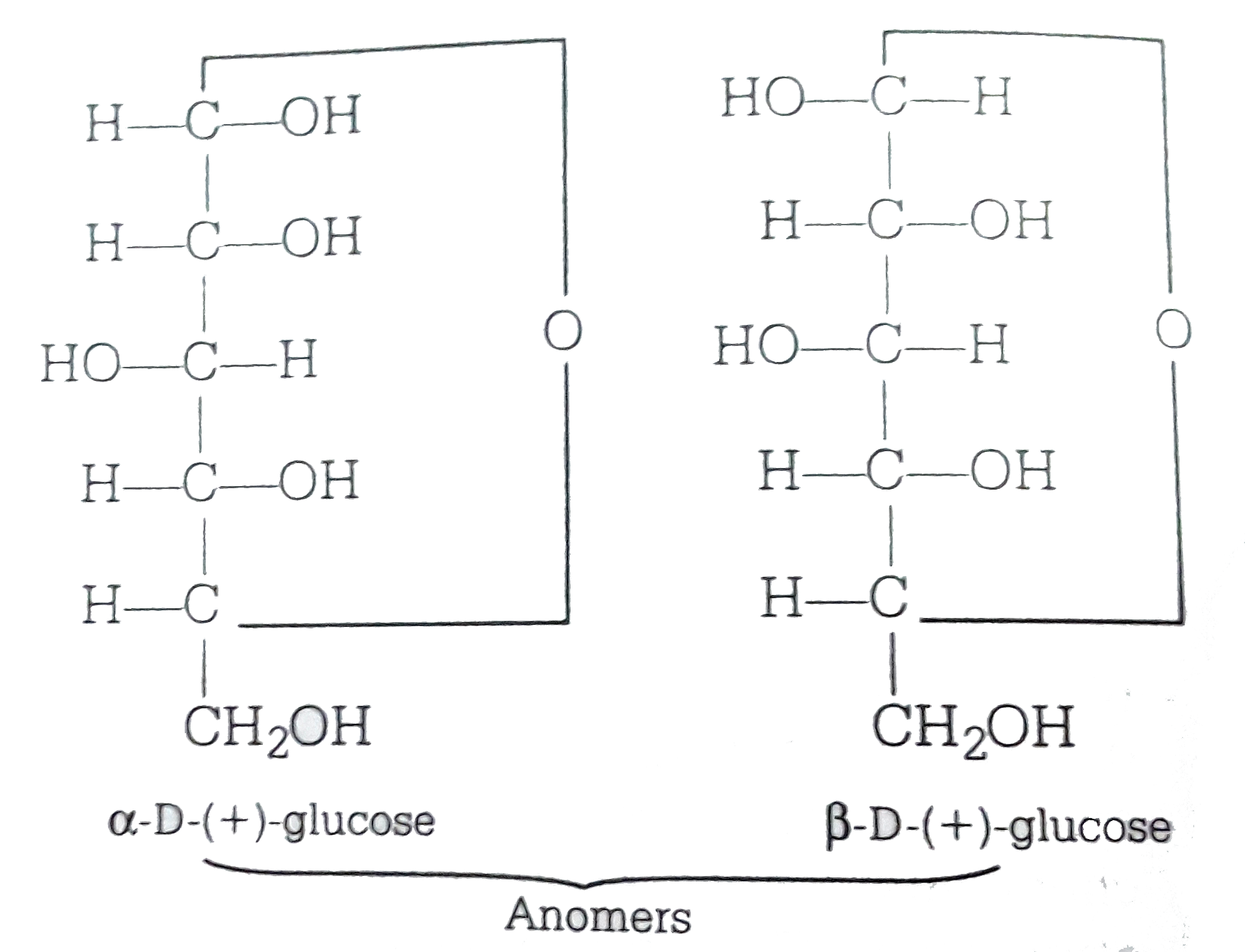
Alpha D Glucose and Beta D Glucose Are Enantiomers
The Epimers of glucose involve some formations, some examples are starch, glycogen, glucose, polysaccharides, and oligosaccharides. The stereoisomers β-D-mannopyranose and β-D-glucopyranose are known as epimers because they differ only in the C-2 position of stereochemistry. The hydroxyl group in the β-D-glucopyranose molecule is equatorial.