Chemical Structure Of Acetic Acid
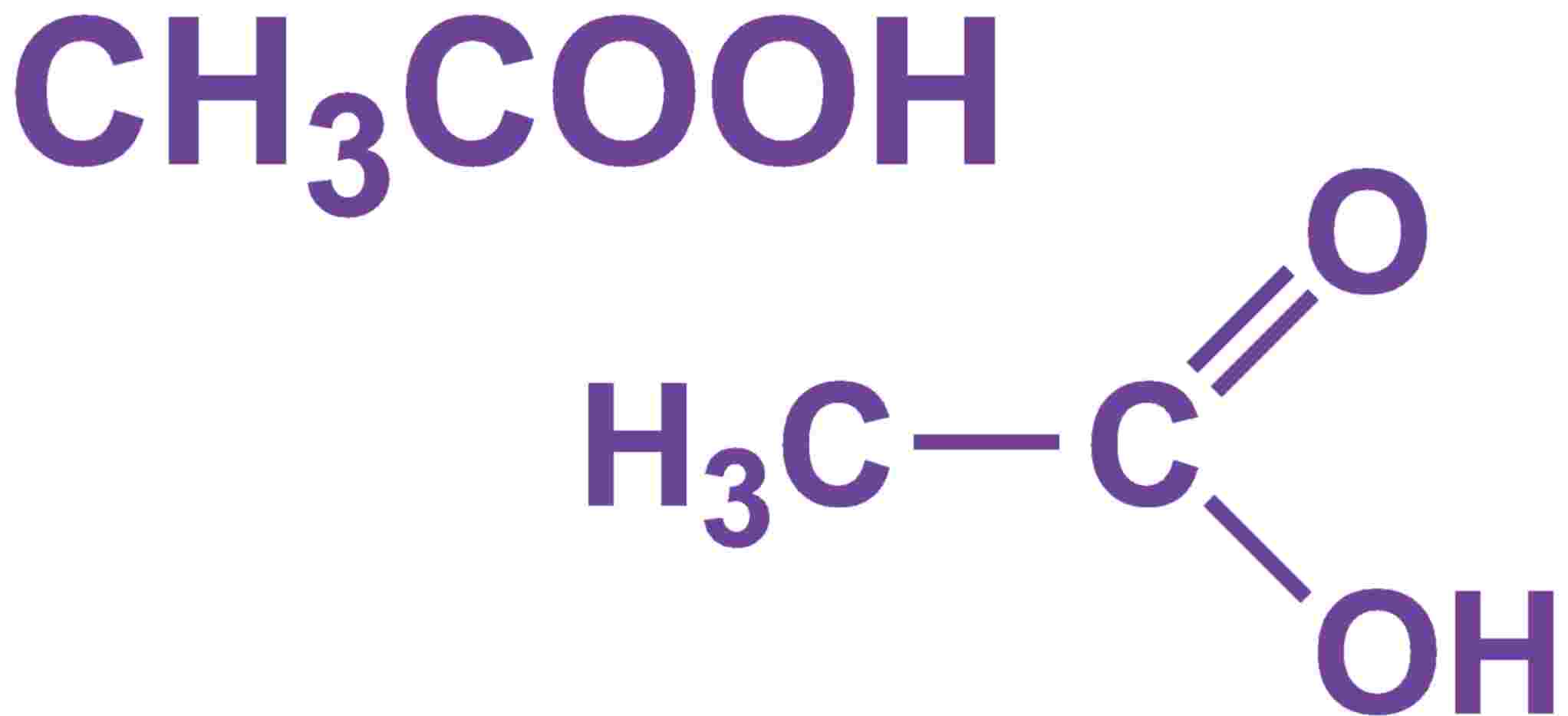
Tổng hợp 85+ hình về mô hình phân tử axit axetic NEC
CH3COOH molecular weight. Molar mass of CH3COOH = 60.05196 g/mol. This compound is also known as Acetic Acid. Convert grams CH3COOH to moles. or. moles CH3COOH to grams. Molecular weight calculation: 12.0107 + 1.00794*3 + 12.0107 + 15.9994 + 15.9994 + 1.00794.

Is Acetic Acid a Strong Acid? Techiescientist
Acetic Acid (CH3COOH)- Acetic Acid is an organic compound with formula CH3COOH.Vinegar is a water solution of acetic acid containing 5-8% of acetic acid by volume. It has a pungent smell and a sour taste. To Learn about the structure of Acetic acid, its preparations , chemical, physical properties, uses and FAQs. Visit BYJU'S for more content.
How to Find the Number of Atoms in CH3COOH (Acetic acid YouTube
The molar mass will be equal to: (1 atom x 56 grams/mole Fe) + (2 atoms x 35.5 grams/mole of chlorine) = 127 grams/mole of iron (II) chloride. For other compounds, this might get a little bit more complicated. For example, take the example of zinc nitrate, or Zn (NO 3) 2. In this compound, we have one atom of zinc, two atoms of nitrogen (one.

Larutan CH3COOH (Mr CH3COOH = 60) dengan kadar 15 dan ma...
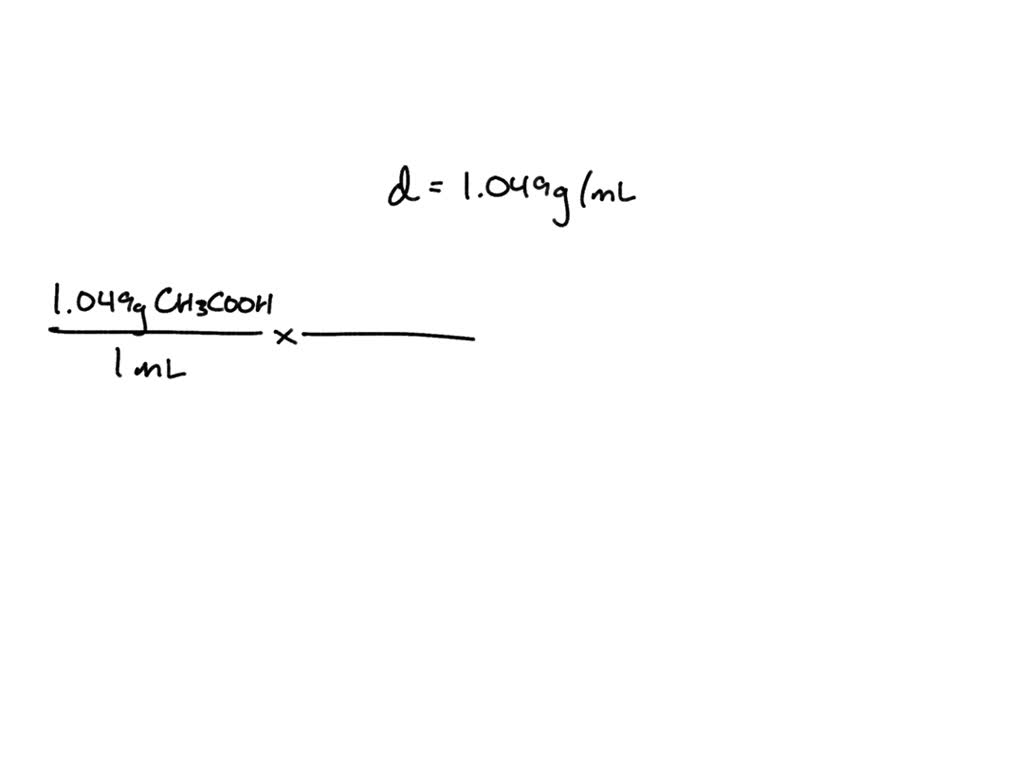
Chemistry. Chemistry questions and answers. Question 34 What is the molarity of glacial acetic acid (CH3COOH, Mr = 60.05 g/mol) at 25°C given that the density of acetic acid at that temperature is 1.049 g/mL? [1] 0.0174 M [2] 0.057 M [3] 57.2 M [4] 60.1 M [5] None of the above Question 35 What is the molarity of a KF (aq) solution containing.
Is CH3COOH (Acetic acid) an Acid, Base, or Neutral YouTube
Explanation of how to find the molar mass of CH3COOH: Acetic acid.A few things to consider when finding the molar mass for CH3COOH:- make sure you have the c.
SOLVED What is the molarity of glacial acetic acid ( CH3COOH, Mr =60.05 g/mL ) at 25 degrees
Molar Mass, Molecular Weight and Elemental Composition Calculator. Molar mass of CH3COOH (vinegar) is 60.0520 g/mol. Get control of 2022! Track your food intake, exercise, sleep and meditation for free. Convert between CH3COOH (vinegar) weight and moles. Compound.
Gels Free FullText Removal of Acetic Acid from Bacterial Culture Media by Adsorption onto a
PUGVIEW.ServerBusy Too many requests or server too busy. Acetic Acid | CH3COOH or C2H4O2 | CID 176 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity information, supplier lists, and more.
Acetic Acid Overview, Structure, Properties & Uses
The formula for molecular mass of Ethanoic acid. The formula for Ethanoic acid is CH 3 COOH. The molecular mass of CH 3 COOH = 2×The atomic mass of C + 4× The atomic mass of H + 2×Atomic mass of O. Step3. Calculation of molecular mass of Ethanoic acid. The molecular mass of Ethanoic acid= 2×12.01+ 4×1.0079+2×16 u. = 60. 0516 u.
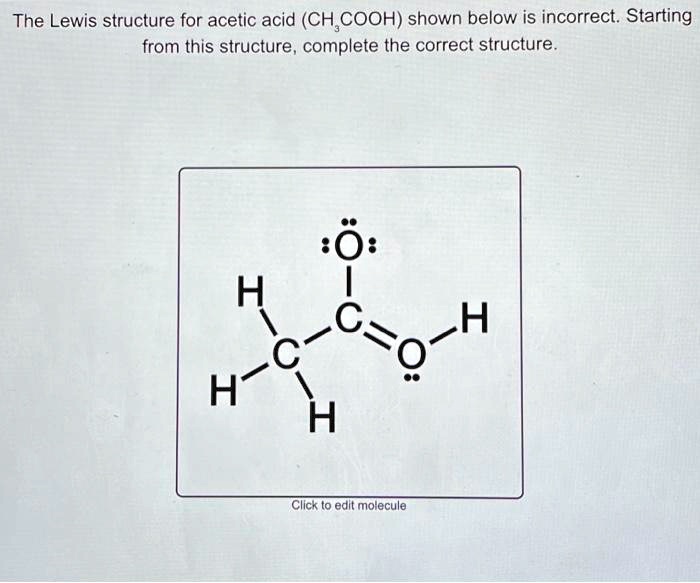
SOLVED The Lewis structure for acetic acid (CH3COOH) shown below is incorrect. Starting from
Click here:point_up_2:to get an answer to your question :writing_hand:calculate the molecular mass of ethanoic acid ch3cooh atomic masses c

Chemical Structure Of Acetic Acid
3. Compute Mass of Each Element. Multiply the number of atoms by the atomic weight of each element found in steps 1 and 2 to get the mass of each element in CH3COOH: Molar Mass (g/mol) C (Carbon) 2 × 12.0107 = 24.0214. H (Hydrogen) 4 × 1.00794 = 4.03176. O (Oxygen)
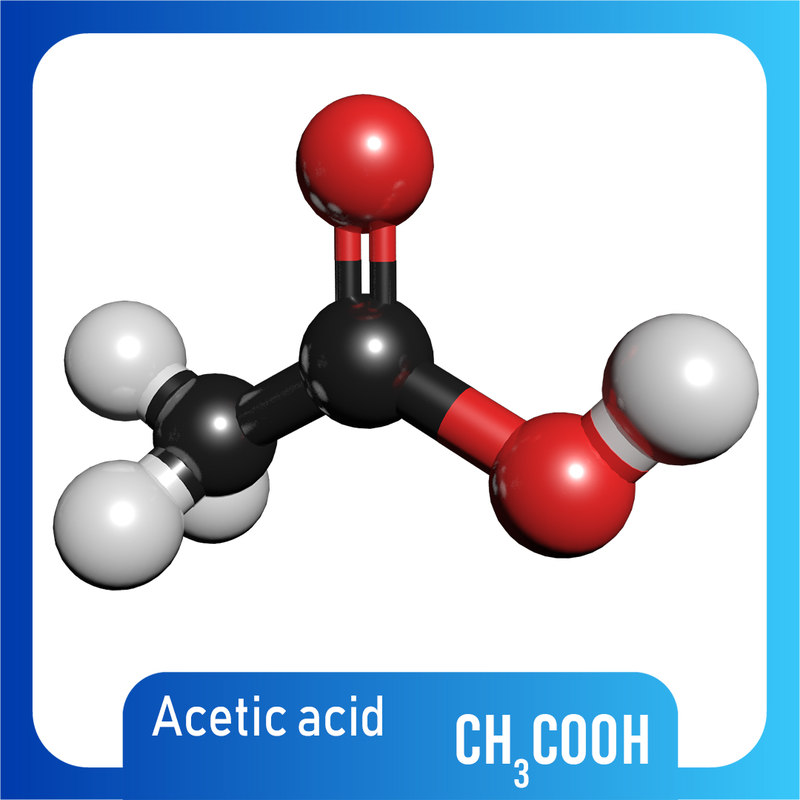
modelo 3d Acido acético 3D CH3COOH Modelo 3D TurboSquid 1420748
Ethanoic Acid - The chemical formula of ethanoic acid is CH3COOH. It is commonly called acetic acid. After adding 5-8% of acetic acid in water it becomes vinegar. Understand its properties, structure, chemical reactions like Esterification, Uses with FAQs of Ethanoic acid. Visit BYJU'S for more information.

Ch3cooh Molecular Geometry
Known values. Density of glacial acetic acid. 1.049 g/ml. Molar mass of acetic acid (CH3COOH) 60.05 g/mole. Concentration of glacial acetic acid. 99.7% (% by mass, wt/wt) Step 1: Calculate the volume of 100 grams of glacial acetic acid solution.Formula: D e n s i t y = w e i g h t v o l u m e.
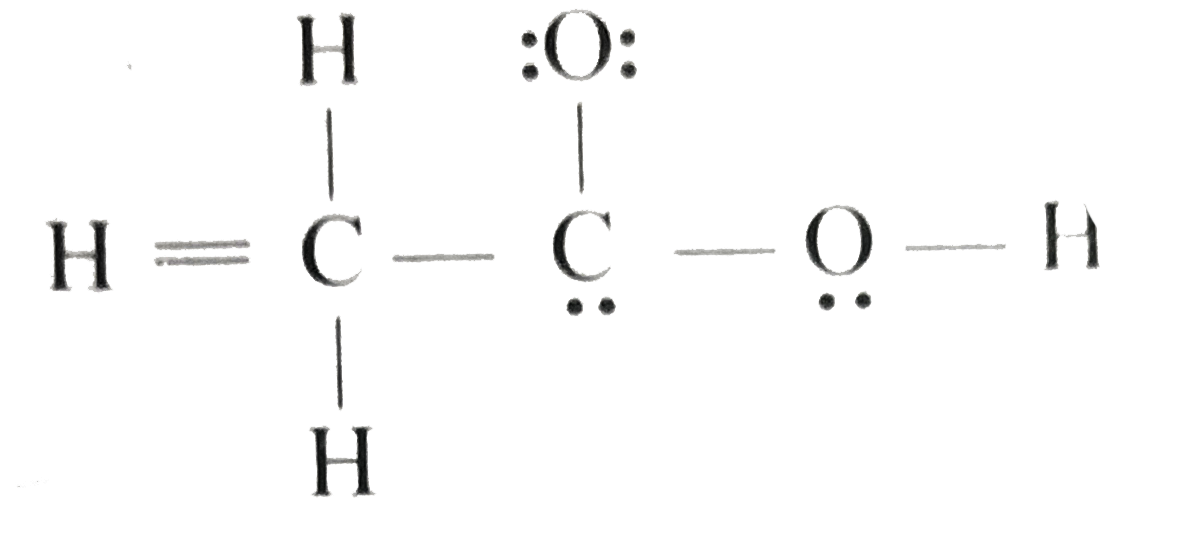
Doubt Solutions Maths, Science, CBSE, NCERT, IIT JEE, NEET
Computing molar mass step by step. First, compute the number of each atom in CH 3 COOH: C: 2, H: 4, O: 2. Then, lookup atomic weights for each element in periodic table: C: 12.0107, H: 1.00794, O: 15.9994. Now, compute the sum of products of number of atoms to the atomic weight: Molar mass (CH 3 COOH) = ∑ Count i * Weight i =.
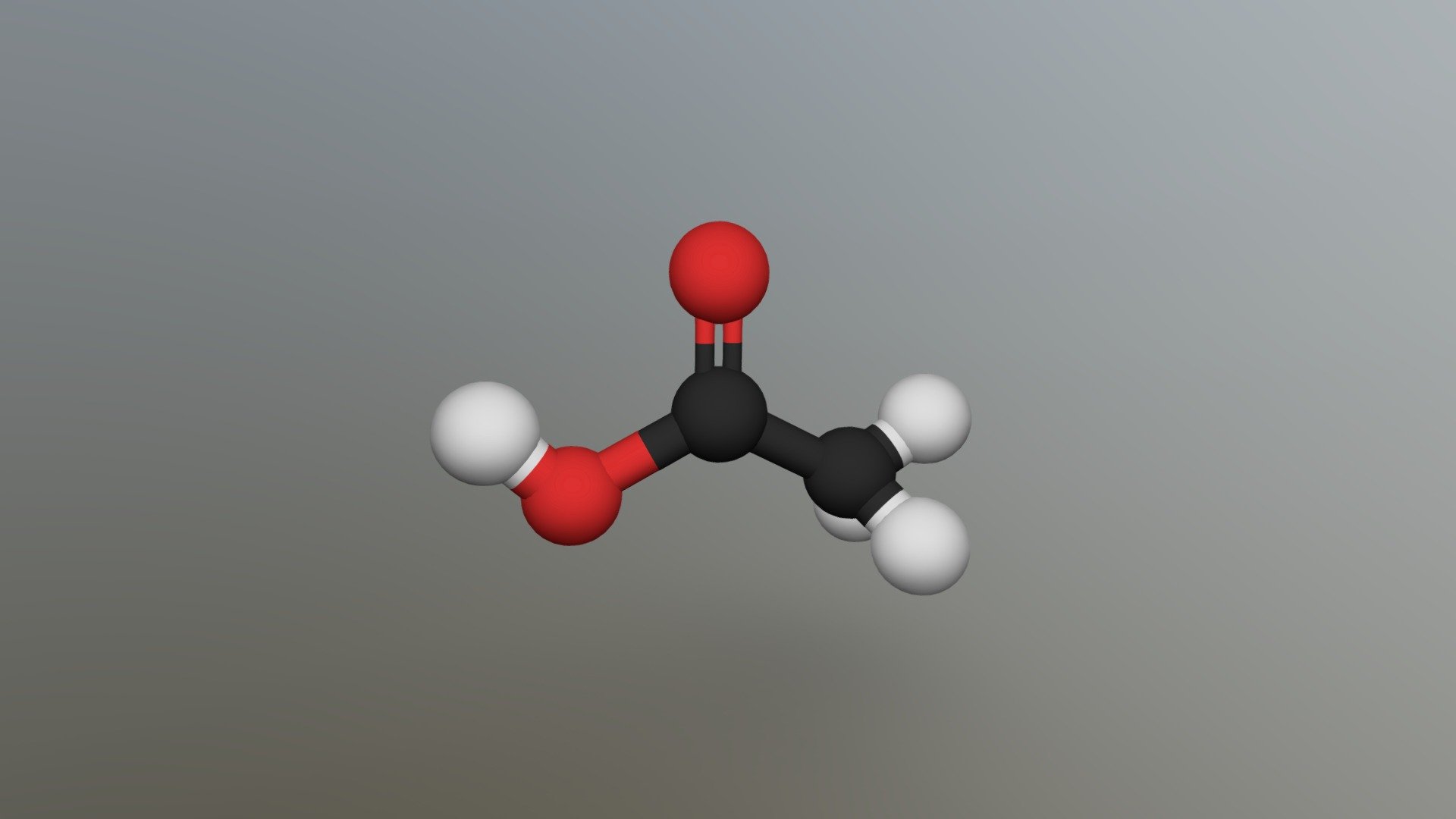
Ch3cooh Molecular Geometry
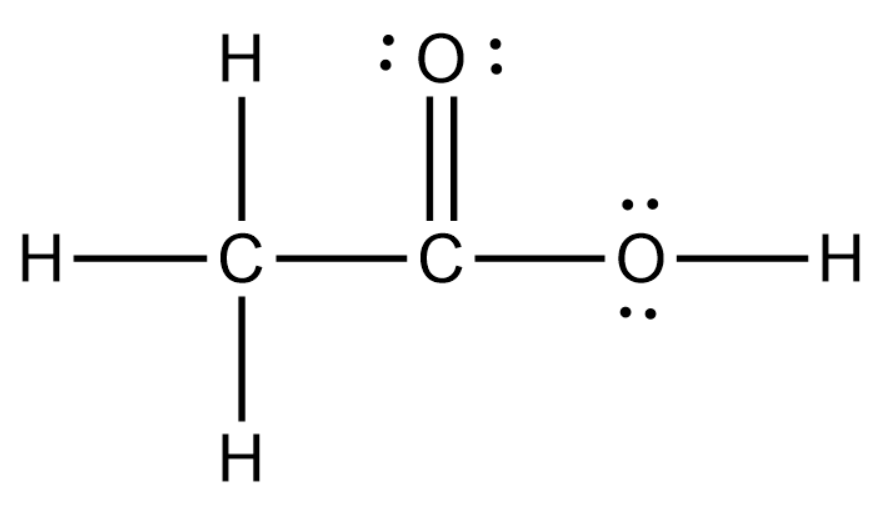
Acetic acid / ə ˈ s iː t ɪ k /, systematically named ethanoic acid / ˌ ɛ θ ə ˈ n oʊ ɪ k /, is an acidic, colourless liquid and organic compound with the chemical formula CH 3 COOH (also written as CH 3 CO 2 H, C 2 H 4 O 2, or HC 2 H 3 O 2). Vinegar is at least 4% acetic acid by volume, making acetic acid the main component of vinegar apart from water. It has been used, as a.
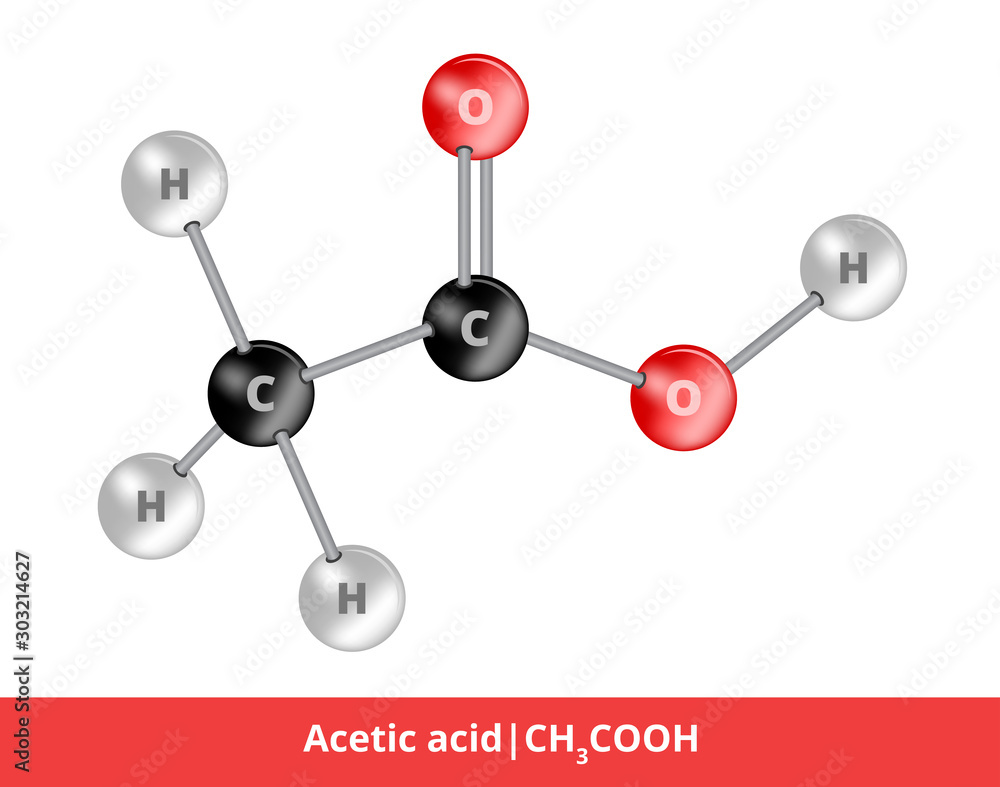
Obraz Vector ballandstick model of organic compound. Icon of acetic acid CH3COOH structure
Pertanyaan. Massa atom molekul dari asam cuka ( CH 3 COOH ) =.. jika diketahui A r H = 1 ; O = 16 ; dan C = 12 .
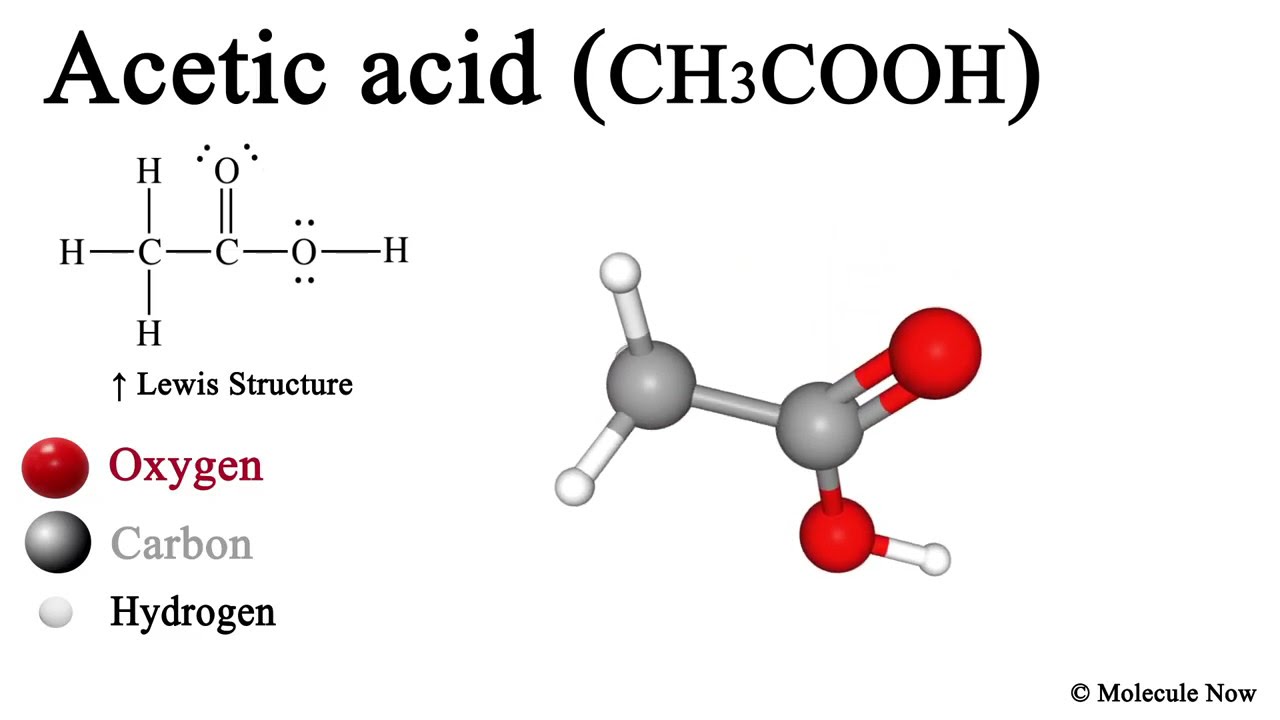
Acetic Acid (CH3COOH) 3D Model with Lewis Structure YouTube
Compute Mass of Each Element. Multiply the number of atoms by the atomic weight of each element found in steps 1 and 2 to get the mass of each element in CH3COOH {-}: Molar Mass (g/mol) C (Carbon) 2 × 12.0107 = 24.0214. H (Hydrogen) 4 × 1.00794 = 4.03176. O (Oxygen)